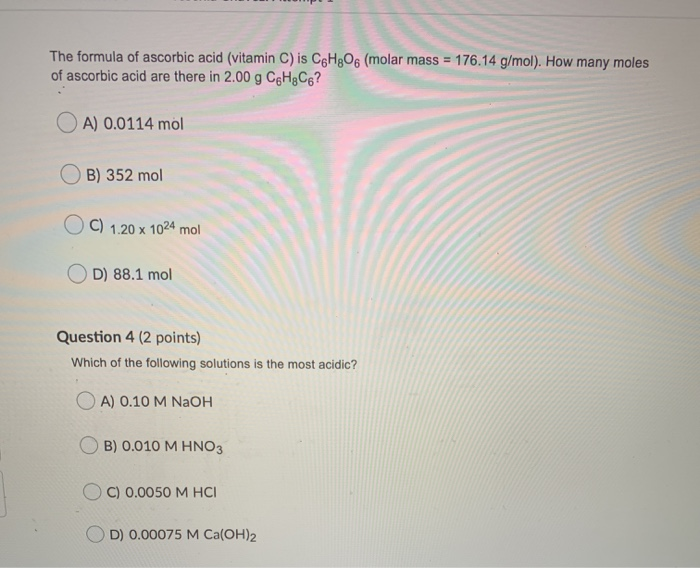
SOLVED: The molecular formula of ascorbic acid (vitamin C) is C6H8O6. Determine the percent composition by mass in vitamin C (the percent of each constituent element).

NCERT Solution Intext 2.11: Calculate the mass of ascorbic acid (Vitamin C, C6H8O6) to be dissolve - YouTube

Chapter Three: Stoichiometry: Calculations with Chemical Formulas and Equations Chapter Three: Stoichiometry: Calculations with Chemical Formulas and Equations. - ppt download
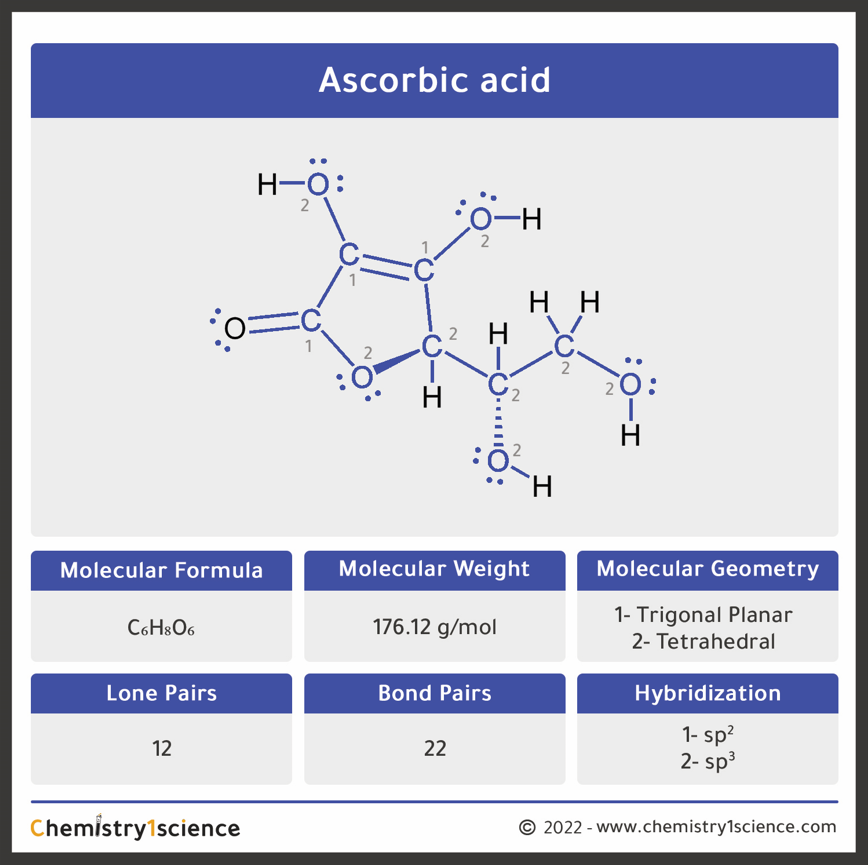
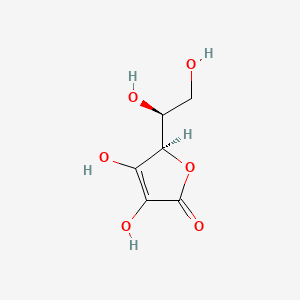
Ascorbic acid (vitamin C) C₆H₈O₆ : Molecular Geometry - Hybridization - Molecular Weight - Molecular Formula - Bond Pairs - Lone Pairs -

SOLVED:Vitamin A has a molar mass of 286.4 g/mol and a general molecular formula of Cx Hy E, where E is an unknown element. If vitamin A is 83.86 % C and
Calculate the mass of ascorbic acid (Vitamin C, C6H8O6) - Sarthaks eConnect | Largest Online Education Community

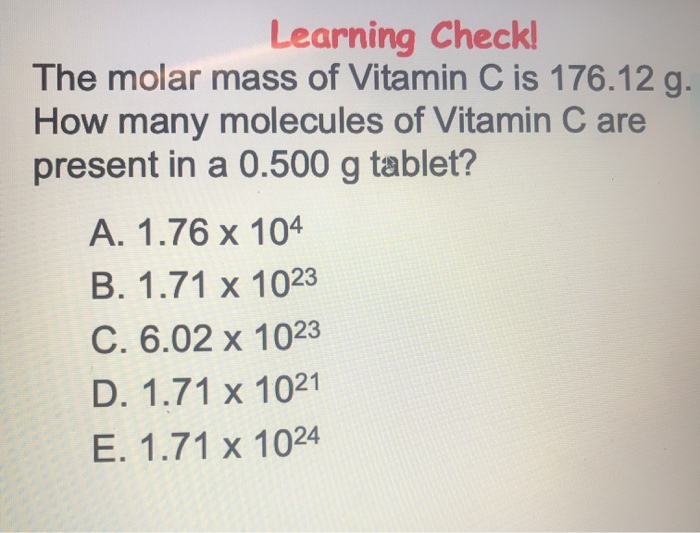
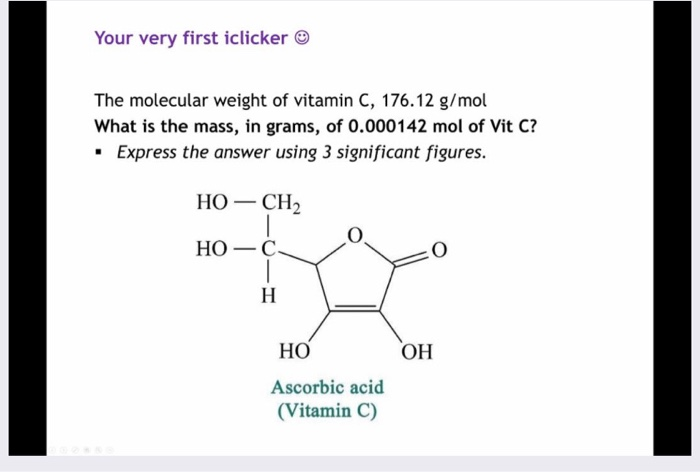
SOLVED: 'What is the mass, in grams, of 0.000142 mol of vitamin C? The molecular weight of vitamin C, C6H8O6, is g 176.12 mol What is the mass, in grams, of 0.000142

SOLVED: Ascorbic acid, vitamin C, is a water-soluble vitamin. Its chemical formula is C6H8O6 and its molar mass is 176.07 g / mol. A solution containing 80.5 g of vitamin C dissolved
Calculate the mass of ascorbic acid (Vitamin C, C6H8O6) to be dissolved - Sarthaks eConnect | Largest Online Education Community

Calculate the mass of ascorbic acid (Vitamin C, C6H8O6) to be dissolved in 75g of acetic acid to.... - YouTube
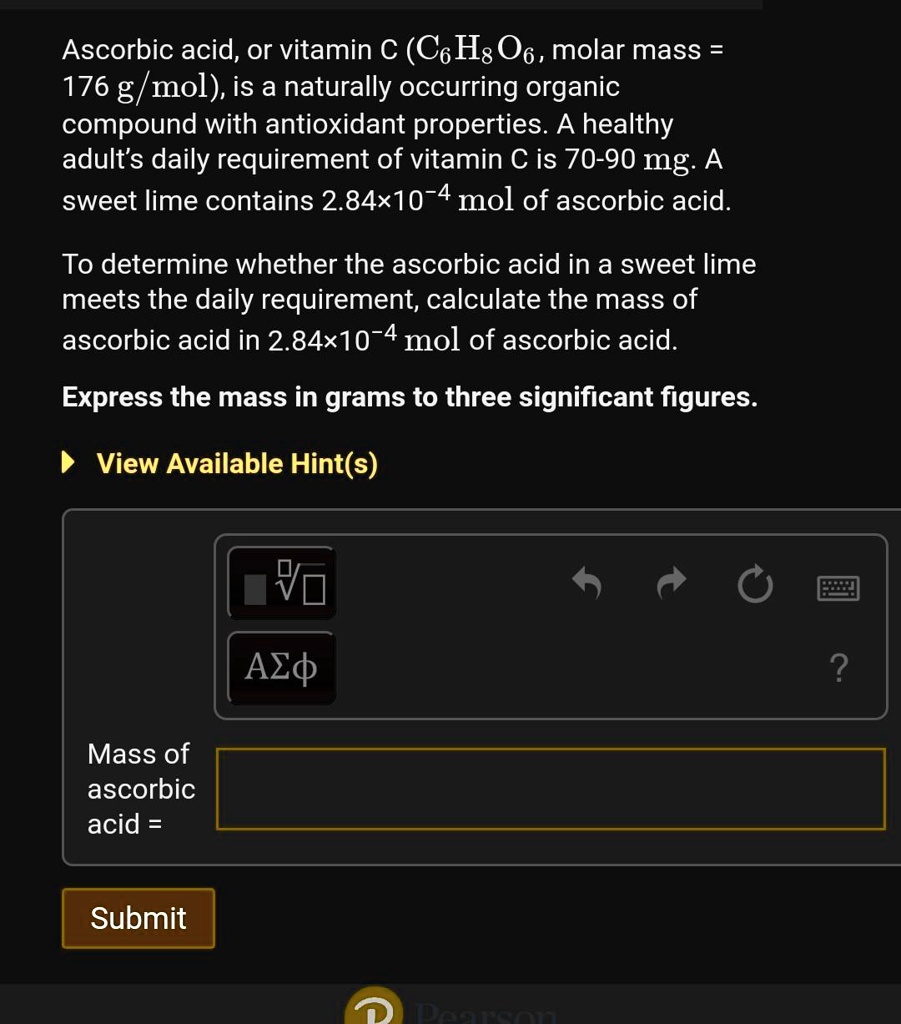
SOLVED: Ascorbic acid; or vitamin € (C6Hs06, molar mass 176 g/mol) is a naturally occurring organic compound with antioxidant properties. A healthy adult's daily requirement of vitamin C is 70-90 mg: A

Calculate the mass of ascorbic acid (Vitamin C. C6H8O6 ) to be dissolved in 75 g of acetic acid to lower its melting point by 1.5^∘C. Kf = 3.9K kg mol^- 1 .

Calculate the mass of ascorbic acid (Vitamin C, C6H8O6) to be dissolved in 75g of acetic acid to.... - YouTube
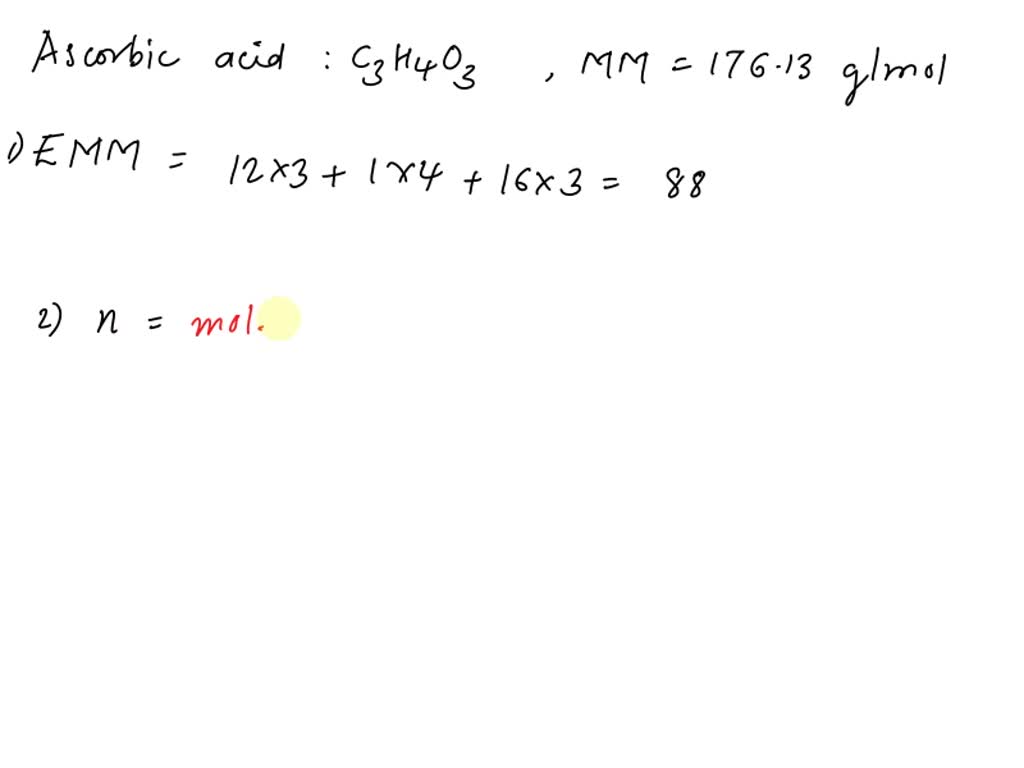
SOLVED: 1. Ascorbic acid has the empirical formula of C3H4O3 and a molar mass of 176.13 g/mol. Determine its: (a) empirical formula molar mass (b) molecular formula. Show your work.

SOLVED: The molar mass of ascorbic acid (vitamin C) is 176.12 g/mol. Ascorbic acid contains C, H, and 0. Mass percent composition analysis shows that this compound contains 40.92% C, 4.58% H