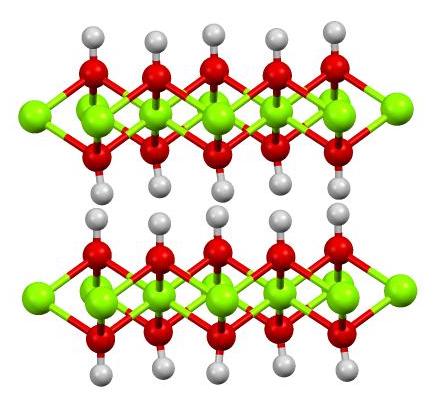
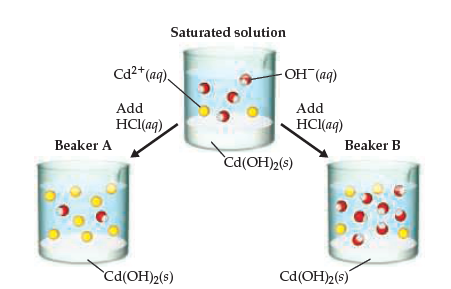
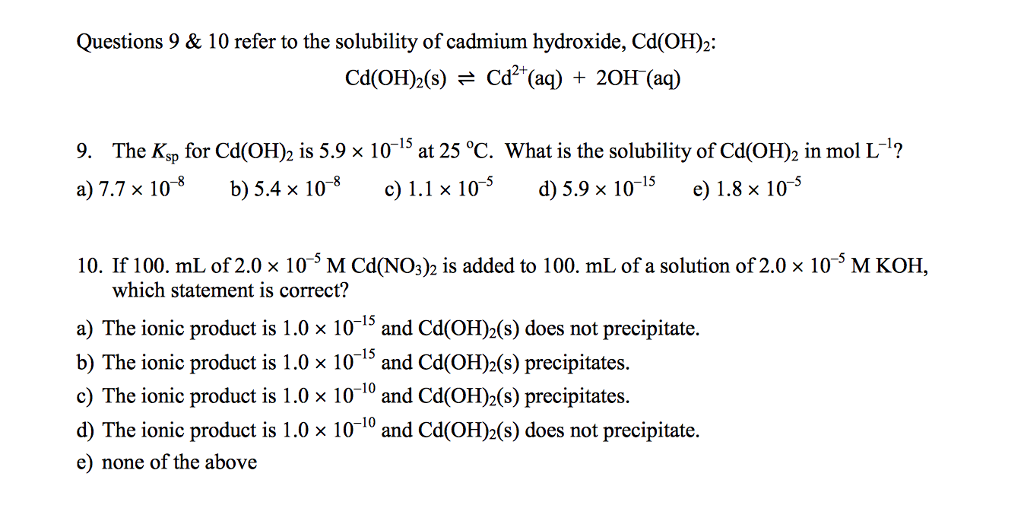
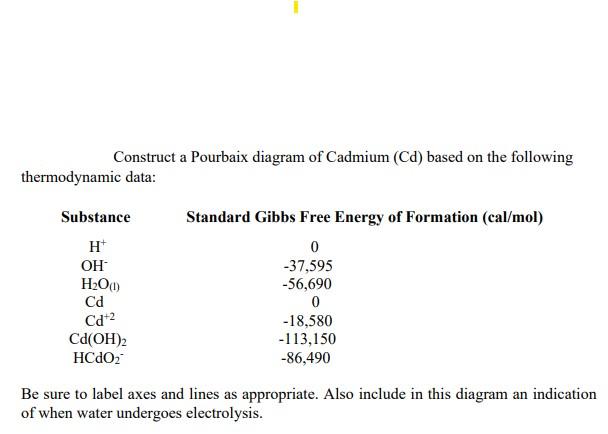
Synthesis of cadmium hydroxide nanostructure via composite-hydroxide-mediated approach - J Adnan, M Arfan, T Shahid, MZ Khan, R Masab, AH Ramish, S Ahtasham, AG Wattoo, M Hashim, A Zahoor, MF Nasir, 2019

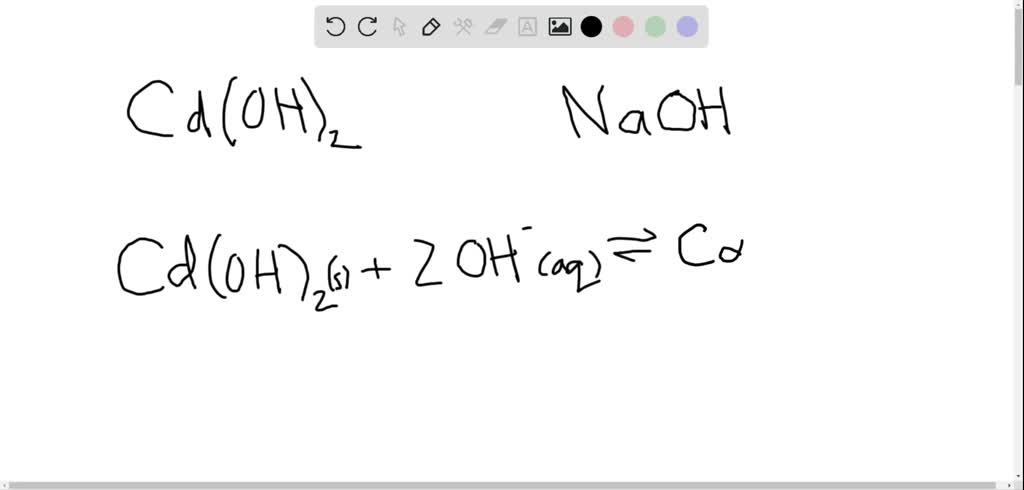
Solubility of `Cd(OH)_(2)` in pure water is `1.84xx10^(-5)\"mole\"//L` Calculate its solubility in - YouTube
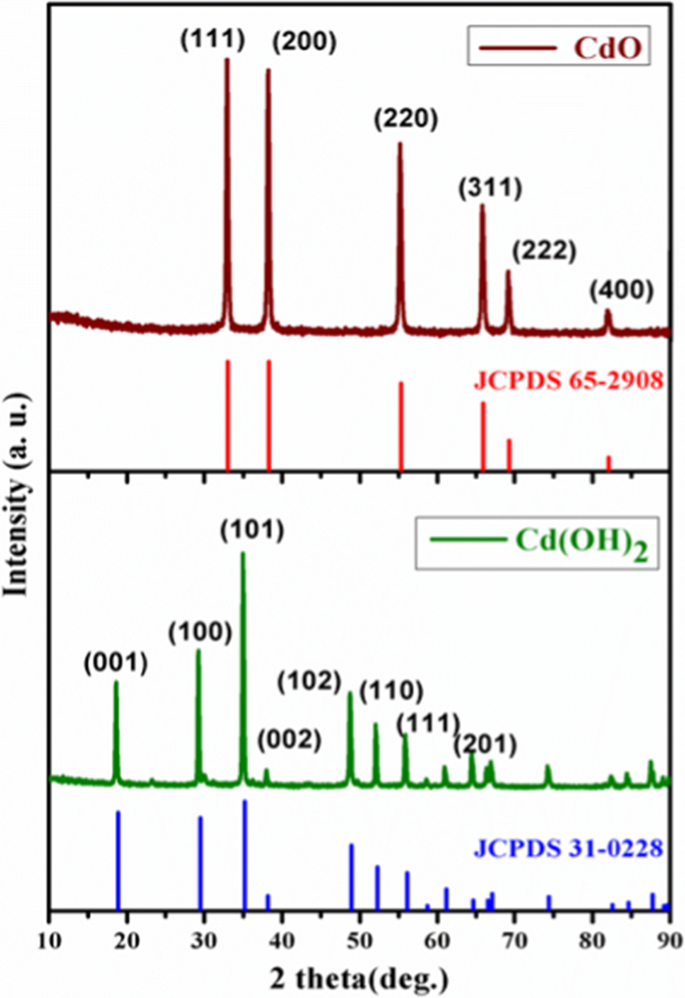
Cd(OH)2 and CdO: structural, optical, electron density distribution analysis with antibacterial assay | SpringerLink
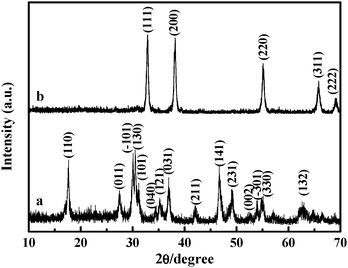
One-step fabrication of Cd(OH)2 nanorings via a solution phase synthesis - Chemical Communications (RSC Publishing) DOI:10.1039/C0CC00665C
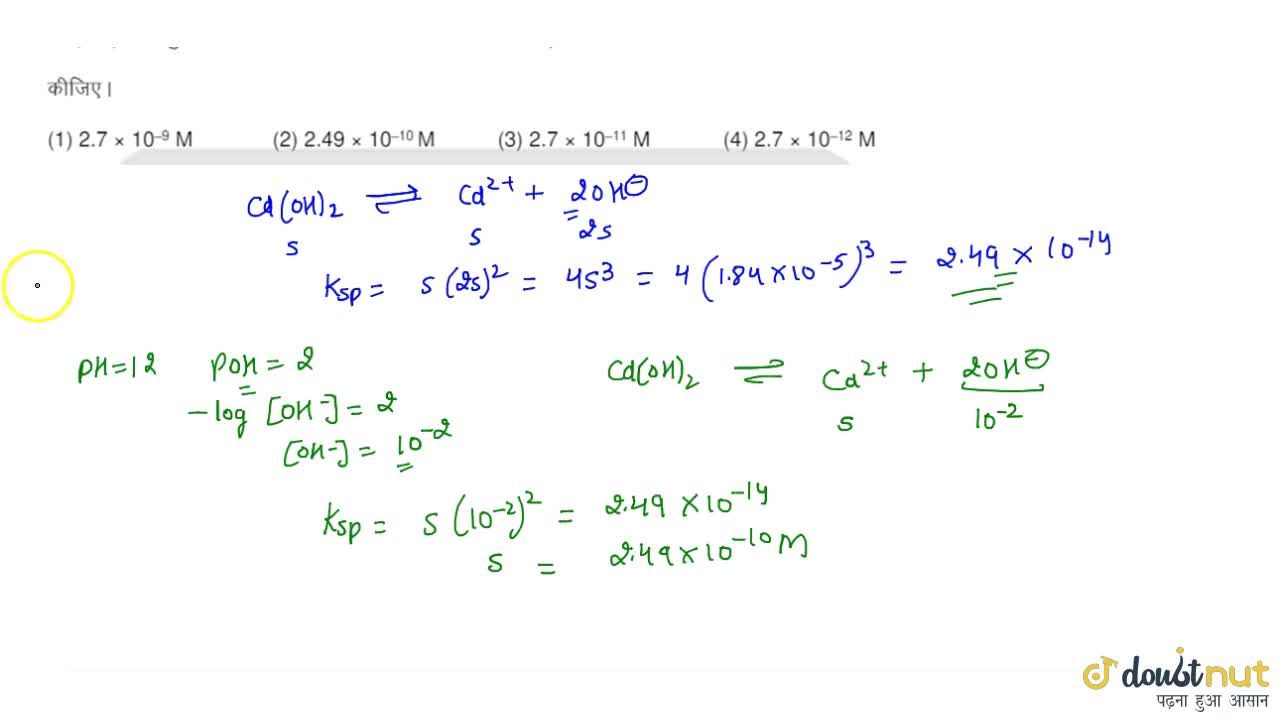
Solubility of Cd(OH)(2) in pure water is 1.84xx10^(-5)"mole"//L Calculate its solubility in a buffer solution of ph=12.